In 2019, 4.95 million people died from antimicrobial resistance. “Even before COVID, which worsened the problem, studies showed that by 2050, the number of deaths by antibiotic resistance will be 10 million,” said Angela Violi, a professor of six studies and U-M Arthur F. Thurnau Professor of mechanical engineering.
A new computer model has been developed at the University of Michigan to help find ways to stop antibiotic-resistant infections and new viruses, and assist in the design of nanoparticles for a variety of treatment purposes.
More information is also available in the article, Nanobiotics: AI for discovering where and how nanoparticles bind with proteins, by Kate McAlpine.
The computer model, NeCLAS, uses AI machine learning techniques to absorb the structural models of proteins and their interaction sites. Once it has this information, it learns how these proteins and nanoparticles might interact, and is able to learn and predict binding sites and their likelihoods, as well as predicting the interactions between two select proteins or nanoparticles.
This is an important step in being able to design antibiotics and antivirals on demand.
Other models exist but NeCLAS, “goes beyond,” said U-M chemical engineering doctoral student Jacob Saldinger, “by showing how nanostructures will interact with one another, and it’s not limited to proteins. This enables researchers to understand the potential applications of nanoparticles and optimize their designs.”
“Besides being more general, NeCLAS also uses way less training data,” said electrical and computer engineering doctoral student Matt Raymond. “We only have 21 nanoparticles to look at, so we have to use protein data in a clever way.”
The team tested three case studies for which they had additional data: using binds as molecular tweezers, how graphene quantum dots break up the biofilm produced by staph bacteria, and whether graphene quantum dots would disperse in water.
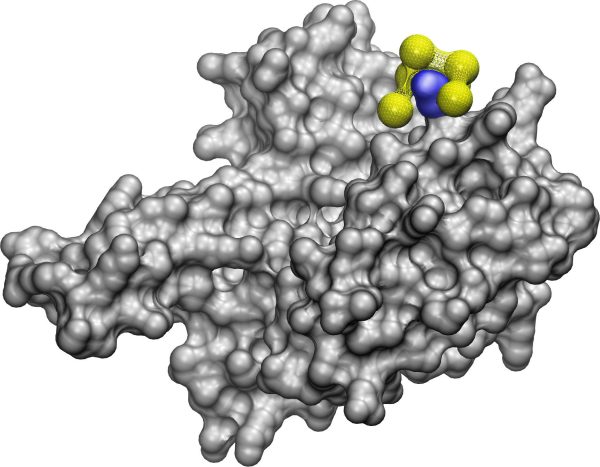
“Without experimental data, you don’t have a starting point for how these proteins and nanoparticles will interact,” said Chloe Luyet, a U-M doctoral student in the Violi group. “NeCLAS can tell me which parts should be closer together at the beginning of the simulation.”
Next, the team intends to explore other biofilms and microorganisms, including viruses.
Article summarized from Michigan Engineering: Nanobiotics: AI for discovering where and how nanoparticles bind with proteins.
